Question
The USDA recently approved soybean with stacked glyphosate/isoxaflutole tolerance. A 1998 EPA report designated isoxaflutole as a "probable carcinogen." What is the environmental/toxicological impact of this USDA decision?
Submitted by: KarthikAghoram
Answer
Expert response from Joe Breier, Ph.D.
Regulatory Toxicologist, Bayer Crop Science
Monday, 14/04/2014 14:37
In approving genetically modified crops, such as soybeans, with dual herbicide tolerance, USDA evaluates the safety of the crop, while EPA assesses the safety of the pesticide used on the crop, which includes consideration of the potential risk of an adverse health effect, such as cancer. Based on exposure estimates provided by EPA (2011), the likelihood that humans are exposed to carcinogenic levels of isoxaflutole (IFT) is extremely low. First and foremost, it should be emphasized that the cancer classification noted above is an indication of cancer hazard only and does not provide insight into cancer risk. The cancer classification for IFT is based on the increased incidence of liver tumors in rats and mice and thyroid tumors in male rats after daily dietary exposure at extremely high dose levels for their entire life. In its most recent dietary assessment (food and drinking water) for IFT, EPA (2011) estimated that exposure for the total U.S. population is approximately 0.000072 mg/kg body weight per day, or less than 0.0000002 ounces per day for an average-size adult weighing about 150 pounds. A daily exposure of 0.0000002 ounces is equivalent to consuming 1/1000th of a drop of water per day—in other words, an extremely tiny amount. In comparison, the dose of IFT that caused tumors in rats is more than seven million times higher than the estimated human exposure. As IFT is applied to crops early in the growing season, practically no residues of the chemical or its metabolites are present in the crop at the time of harvest, and thus exposure to humans via the diet is negligible. In addition, EPA (2011) has conducted an aggregate exposure assessment taking into account all possible routes of exposure for all existing and proposed uses for IFT and concluded that the potential increase in cancer risk for the U.S. population due to exposure to IFT is negligible.
Reference:
U.S. Environmental Protection Agency. (2011). Isoxaflutole. Section 3 Registration for Use on Soybeans. Human-Health Risk Assessment. Health Effects Division (HED), Office of Chemical Safety and Pollution Prevention. PC Code: 123000, DP No.: D382796. 09 September 2011.
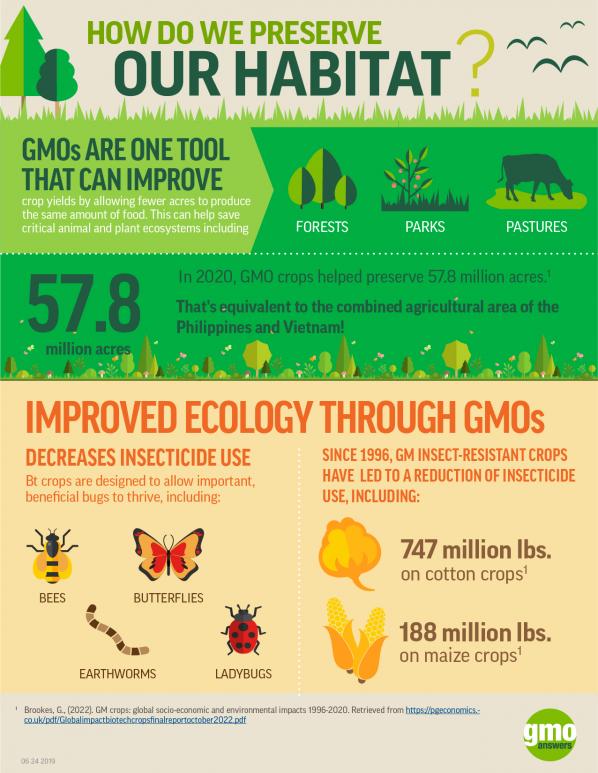
How Do GMOs Benefit The Environment?